1. Describe a chemical test for water.
There are two ways in which you can go about. The first is using anhydrous copper sulphate; in the presence of water, the anhydrous copper sulphate turns from white to blue. Alternatively, you could use anhydrous cobalt chloride; in the presence of water, the anhydrous cobalt chloride turns from blue to pink.
2. Describe and explain, in outline, the
purification of the water supply by filtration
and chlorination.
Filtration is used to separate insoluble substances from the water. Afterwards, the water is chlorinated to get rid of bacteria that couldn't be removed through filtration.
3. State some of the uses of water in industry
and in the home.
Home: cooking, cleaning, washing
Industry: cooling, planting in agriculture
4. Describe the separation of oxygen and
nitrogen from liquid air by fractional
distillation.
Like crude oil, liquid air can be separated in a fractionating column. Liquid air is pumped into the bottom of the column and it is heated. Nitrogen gas evaporates from the air and is collected at the top of the column, while liquid oxygen is collected from the bottom.
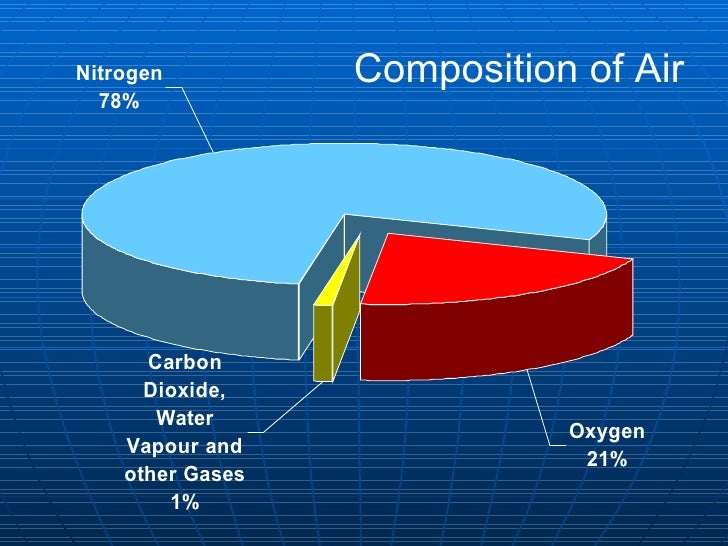
5. Describe the composition of clean air as
being a mixture of 78% nitrogen, 21%
oxygen and small quantities of noble gases,
water vapour and carbon dioxide.
6. State the common air pollutants as carbon
monoxide, sulphur dioxide and oxides of
nitrogen, and describe their sources.
Carbon monoxide, sulphur dioxide and nitrogen oxides are what make air dirty. Carbon monoxide comes from the incomplete burning of fuels, so any machinery that burns a fuel could release carbon monoxide. Additionally, burning cigarettes also produce carbon monoxide. Sulphur dioxide and nitrogen oxides both come from burning fossil fuels.
7. Explain the presence of oxides of nitrogen
in car exhausts and their catalytic removal.
Oxides of nitrogen are formed in car engines due to heat and pressure. They are poisonous and harmful to humans, so catalytic converters are put into engines. Nitrogen oxide is reduced, meaning that it loses oxygen, so it breaks down into separate nitrogen and oxygen gases. In addition, carbon monoxide is oxidised, meaning it gains oxygen, so it becomes carbon dioxide.
8. Explain why the proportion of carbon
dioxide in the atmosphere is increasing, and
why this is important.
Carbon dioxide levels are rising due to excessive human activity, such as factory production and deforestation. This is important because CO2 is one of the gases that contribute to the greenhouse effect, therefore resulting in global warming. Global warming is the general increase of temperature, and this has a great effect on our environment.
9. State the adverse effect of common air
pollutants on buildings and on health.
Air pollutants affect the materials used to build buildings; they cause the materials to become discoloured, brittle, and prone to deterioration. Pollutants also have negative effects on health, including eye, throat and nose irritation, breathing problems, and cancer.
10. Describe the formation of carbon dioxide:
• as a product of complete combustion of
carbon-containing substances,
• as a product of respiration,
• as a product of the reaction between an
acid and a carbonate,
• as a product of thermal decomposition.
When anything containing carbon burns, the carbon reacts with oxygen, gets oxidised, and becomes carbon dioxide. Carbon dioxide is a product of respiration, from the equation glucose + oxygen --> carbon dioxide and water. Similarly, when an acid and carbonate react together, a product of the reaction is carbon dioxide (the other is water). Carbon dioxide is also produced from thermal decomposition. Take the decomposition of calcium carbonate for instance: when calcium carbonate is heated, it breaks down into calcium oxide and carbon dioxide.
11. Describe the essential conditions for the
manufacture of ammonia by the Haber
process including the sources of the
hydrogen and nitrogen, i.e. hydrocarbons or
steam and air.
The Haber process is a process that manufacture ammonia. Nitrogen from the atmosphere and hydrogen, formed through the reacted of methane and steam, are reacted together to form ammonia; this is a reversible reaction.
nitrogen + hydrogen <--> ammonia
N + 3H <--> NH3
The amount of ammonia produced at a time is quite low - about 15%. To produce a higher yield, the following conditions are needed:
• low temperature
• high pressure
• catalyst of iron to speed up the reaction
This is an important process because ammonia is used in the manufacture of fertilisers used in agriculture. Fertilisers speed up the growth of plants and gives them additional nutrients, so that they grow quickly and healthily.
12. Describe the rusting of iron in terms of a
reaction involving air and water, and simple
methods of rust prevention, including paint
and other coatings to exclude oxygen.
Iron rusts when it comes into contact with both air and water. Water reacts with the iron over time and the oxygen in the air oxidises the iron. Combined, this forms a layer of rust.
There are several ways to prevent rust from forming. One would be to simply use a coat of paint; this acts as a barrier to prevent contact with water and air. Another way would be to galvanise the iron, which is to coat the iron in zinc. Similar to paint, the zinc acts as a barrier. Lastly, sacrificial protection can be used. A block of zinc is placed next to the iron, so that any water and air present reacts with the zinc, rather than the iron.
13. Describe the need for nitrogen-,
phosphorus- and potassium-containing
fertilisers.
Plants need nitrogen, phosphorus and potassium to grow healthily. Nitrogen helps with the production of proteins, which is a plant's food. Phosphorus helps balance out the pH of the soil, and potassium helps with the production of chloroplasts, which help the plant photosynthesise.
14. Describe the displacement of ammonia
from its salts by warming with an alkali.
When an ammonium salt is warmed with an alkali, the ammonia in the salt is displaced by ammonia gas.